Abstract
Introduction: Diffuse large B-cell lymphoma (DLBCL) is an aggressive form of non-Hodgkin's lymphoma with high economic burden and a strong pipeline of novel therapies. To better understand this rapidly evolving treatment landscape and current economic burden in DLBCL, this study describes real-world treatment patterns, healthcare resource use (HRU), and costs in DLBCL patients in the US, overall and by line of therapy (LOT).
Methods: Optum Clinformatics claims data from October 2015 to June 2020 were used to identify adult patients with ≥2 diagnosis codes for DLBCL on ≥2 distinct dates within 12 months. The index date was defined as the date of the first observed DLBCL diagnosis. Continuous enrollment was required for ≥12 months pre-index (i.e., baseline period) and ≥1-month post-index. Patients with diagnosis codes for DLBCL or any other malignancy during baseline were excluded to increase the likelihood of capturing newly diagnosed patients. Demographic and clinical characteristics were assessed during baseline, and all-cause and DLBCL-related HRU (monthly incidence rates and 95% confidence intervals [CI]) and costs (per patient per month [PPPM]) were estimated from the index date to the earliest of end of either continuous enrollment or study period. A LOT algorithm was developed to identify lines of National Comprehensive Cancer Network (NCCN)-recommended systemic therapies, stem cell transplantation (SCT), and chimeric antigen receptor (CAR)-T therapies during the study period among a subset of patients with no claims for treatments of interest during the baseline period and ≥1 claim for treatment during the study period. HRU and costs were described during the first line (1L), second line (2L), and third and subsequent line (3L+) periods.
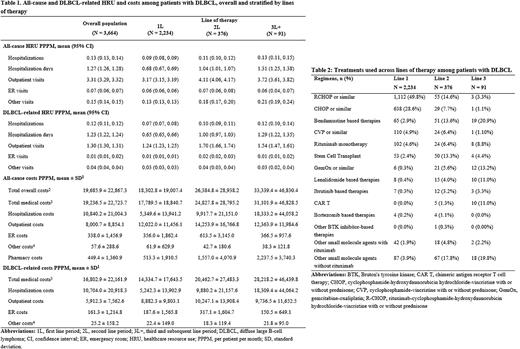
Results: A total of 3,664 patients with DLBCL were included in the study and followed for a mean duration of 18 months post-DLBCL diagnosis. Patients were on average 70 years old, with 47% females, and 75% covered by Medicare. Mean National Cancer Institute Comorbidity Index (NCICI) was 2.2 and the most prevalent comorbidities were cardiovascular disease (32%), diabetes (29%), and chronic obstructive pulmonary disease (24%). On average, patients had 0.13 (95% CI: 0.13, 0.14) all-cause hospitalizations, 3.31 (3.29, 3.32) outpatient visits, 0.07 (0.06, 0.07) emergency room (ER) visits, and 0.15 (0.14, 0.15) other visits PPPM (Table 1). Almost all hospitalizations were DLBCL-related (0.12 PPPM), while outpatient, ER, and other visits were less driven by DLBCL-related visits (1.3, 0.01, and 0.04 visits PPPM respectively). Total all-cause costs were $19,686 PPPM and largely consisted of DLBCL-related medical costs ($16,803). Costs from DLBCL-related hospitalizations accounted for roughly half of the total all-cause costs ($10,704).
A total of 2,234 patients (61%) were included for the treatment pattern analysis after applying additional inclusion criteria. Of those, 376 (17%) received a treatment of interest in the relapsed/refractory setting. Rituximab, cyclophosphamide, hydroxydaunorubicin hydrochloride, vincristine, and prednisone (R-CHOP) was the most common treatment in 1L (50%), followed by CHOP (29%) (Table 2). In the 2L setting, R-CHOP (15%), bendamustine-based therapies (14%), and SCT (13%) were the most common regimens. Bendamustine-based therapies (21%), gemcitabine-based regimens (13%), lenalidomide-based therapies (11%), and CAR-T therapy (11%) were commonly used in 3L. Mean treatment duration decreased with increasing lines of therapy (111 days for 1L, 98 days for 2L, and 81 days for 3L). All-cause and DLBCL-related HRU and costs tended to increase with increasing lines of therapy and were generally highest for 3L+ (Table 1). This was especially true for DLBCL-related hospitalization costs, which increased from $5,242 PPPM in 1L to $18,309 PPPM in 3L+ and accounted for increasingly larger proportions of total all-cause costs with increasing lines.
Conclusions: This study summarizes the current treatment landscape and economic burden for DLBCL in the US. Results show that various therapies are being used for DLBCL management and quantify the high HRU and cost burden among patients with DLBCL, especially during later lines of therapy, highlighting an area of unmet need.
Disclosures
Garg:Merck & Co., Inc.: Current Employment. Satija:Merck & Co., Inc.: Consultancy, Other: I am an employee of Analysis Group, Inc., which received consulting fees from Merck. Song:Merck & Co., Inc.: Consultancy, Other: I am an employee of Analysis Group, Inc., which received consulting fees from Merck; Gamida Cell, Inc.: Consultancy. Sarpong:Merck & Co., Inc.: Current Employment. Meade:Merck & Co., Inc.: Consultancy, Other: I am an employee of Analysis Group, Inc., which received consulting fees from Merck. Lemus-Wirtz:Merck & Co., Inc.: Consultancy, Other: At the time of this research, I was an employee of Analysis Group, Inc., which received consulting fees from Merck. Gaburo:Merck & Co., Inc.: Consultancy, Other: I am an employee of Analysis Group, Inc., which received consulting fees from Merck. Signorovitch:Merck & Co., Inc.: Consultancy, Other: I am an employee of Analysis Group, Inc., which received consulting fees from Merck; Gamida Cell, Inc.: Consultancy. Raut:Merck & Co., Inc.: Current Employment. Ryland:Merck & Co., Inc.: Current Employment. Chakraborty:Merck & Co., Inc.: Current Employment, Current equity holder in private company.
Author notes
Asterisk with author names denotes non-ASH members.